What Staple Oligomers Can Do
- Downregulate disease-causing proteins
- Upregulate the proteins essential for life
- Inhibit viral replication
- Skip (delete) abnormal protein sequences
Distinguishing Features of
Staple Oligomers
- High target gene selectivity with minimal off-target effects
- Excellent in vivo stability
- Broad therapeutic applicability to various diseases
Proteins Essential for Life
Human life activities are maintained by a balanced expression of proteins that compose the body. Extreme decreases or increases in protein expression levels, or the production of abnormal proteins, can disrupt this balance and lead to serious diseases.
How Staple Oligomers Work
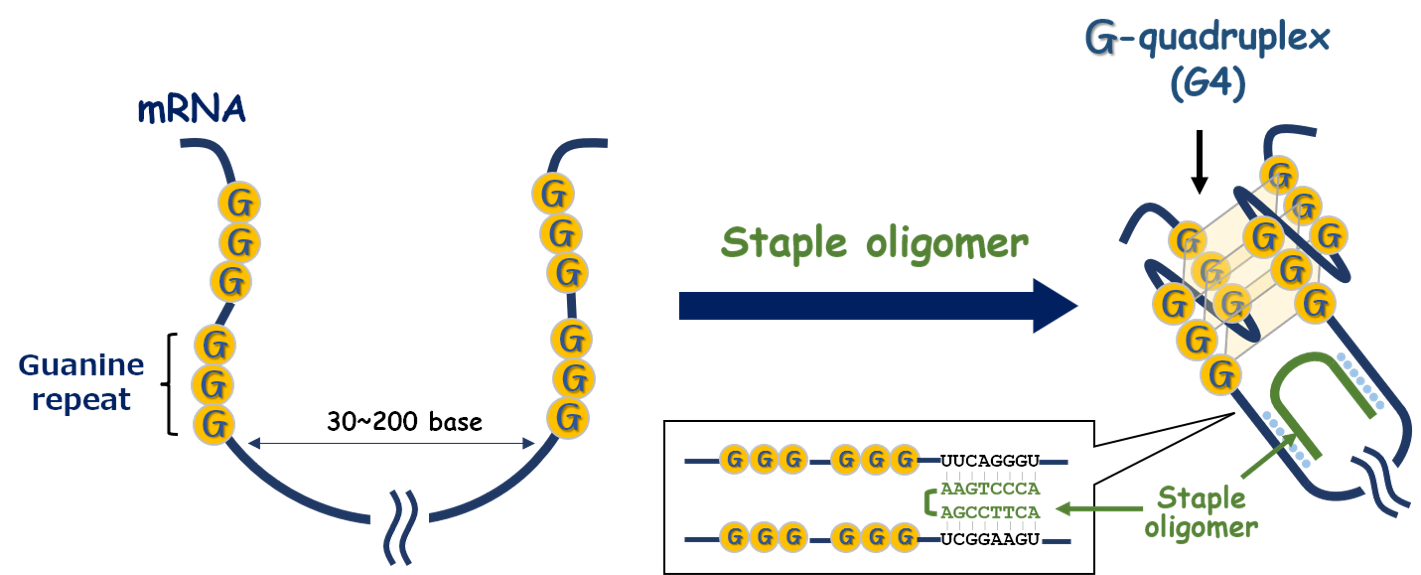
Staple oligomers can improve these imbalances in disease states. Staple oligomers specifically bind to the flanking sequences of guanine repeats in target mRNAs and artificially induce the formation of G-quadruplex (G4) structures by bringing the guanine repeat sequences closer together, thereby modulating protein expression by either increasing or suppressing translation. Additionally, Staple oligomers can normalize abnormal protein expression patterns.
High Target Selectivity and Reduced Off-Target Effects
Staple oligomers are designed for each target gene, ensuring high target selectivity and minimizing off-target effects (side effects from interaction with non-target genes).
Excellent in vivo Stability
It is possible to construct Staple oligomers with metabolically stable artificial nucleic acids. Since their mechanism in independent of intracellular enzymes, their efficacy is not diminished even if the entire sequence is composed of artificial nucleic acids.
Versatility and Rapid Functional Validation
Compared to conventional nucleic acid medicine technologies, Staple oligomers offer greater flexibility in sequence design and enable rapid progression from design to functional validation. Staple oligomers also hold the potential to overcome various challenges faced by existing nucleic acid therapeutics.
Publication
“Staple oligomers induce a stable RNA G-quadruplex structure for protein translation inhibition in therapeutics”
Yousuke Katsuda, Takuto Kamura, Tomoki Kida, Rinka Ohno, Shuhei Shiroto, Yua Hasegawa, Kaito Utsumi, Yuki Sakamoto, Shinichiro Nakamura, Taishi Nakamura, Kenichi Tsujita, Yusuke Kitamura, Yukiko Kamiya, Hiroyuki Asanuma, Toshihiro Ihara, Masaki Hagihara, Shin-Ichi Sato
Nature Biomedical Engineering, 15 October (2025)
doi: 10.1038/s41551-025-01515-4
https://www.nature.com/articles/s41551-025-01515-4

